
Hydrogen is a unique element in that it contains one proton in the nucleus and one electron. In hydrogen gas, two hydrogen nuclei (called proton) come together in close proximity and share two electrons to form a single covalent bond. The electron density between both nuclei is constant and thus the H2 molecule is termed non-polar. The potential energy of the H-H bond is very high at 432 kJ/mol which provides hydrogen with high energy for its low molecular mass, 33.3 MJ.
However, when hydrogen is combined with other elements such as metals, the hydrogen and metal nuclei share electrons, but the uniformity of the electron density is not constant, the bulk of the density is concentrated around the hydrogen nuclei with less around the metal. This is due to differences in electronegativity, where hydrogen (2.20) is more electronegative than most transition metals, magnesium (Mg) shown below. The bond is now termed polar. However, chemists signify this shift of electron density by indicating a partial negative charge at H and a partial positive charge at the metal. Hence the hydrogen in MgH2 is termed a hydride.
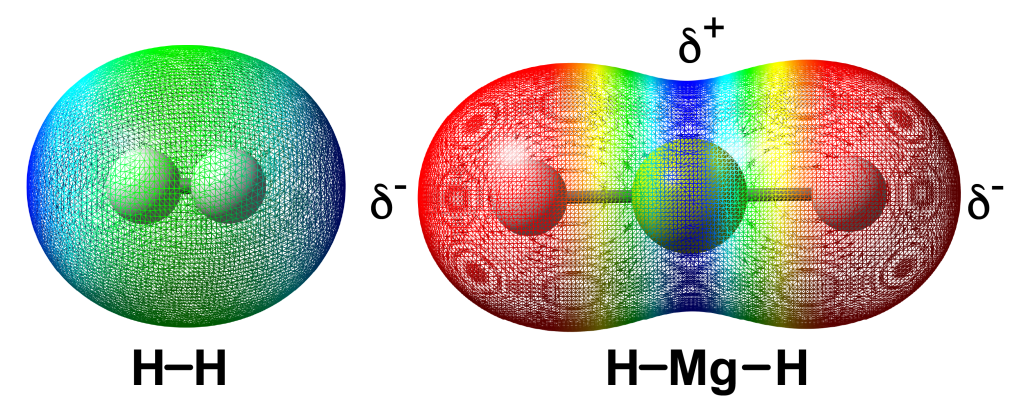
Computational model of electrostatic density maps of (left) dihydrogen and (right) magnesium hydride, in the gas phase.
Hydrides are very powerful reagents and well suited to transfer a hydrogen/electron combination to another molecule. For hydrogen storage purposes, recreating dihydrogen gas requires reaction of a hydride with a proton (H+) source, i.e., a hydrogen atom with no electron. Many naturally occurring molecules can provide a proton (H+) including water, methanol, ethanol, ammonia and many acids like sulphuric acid, hydrochloric acid and acetic acid (vinegar). Notice that the electrostatic density map for water and ammonia are very different from that of a metal hydride.
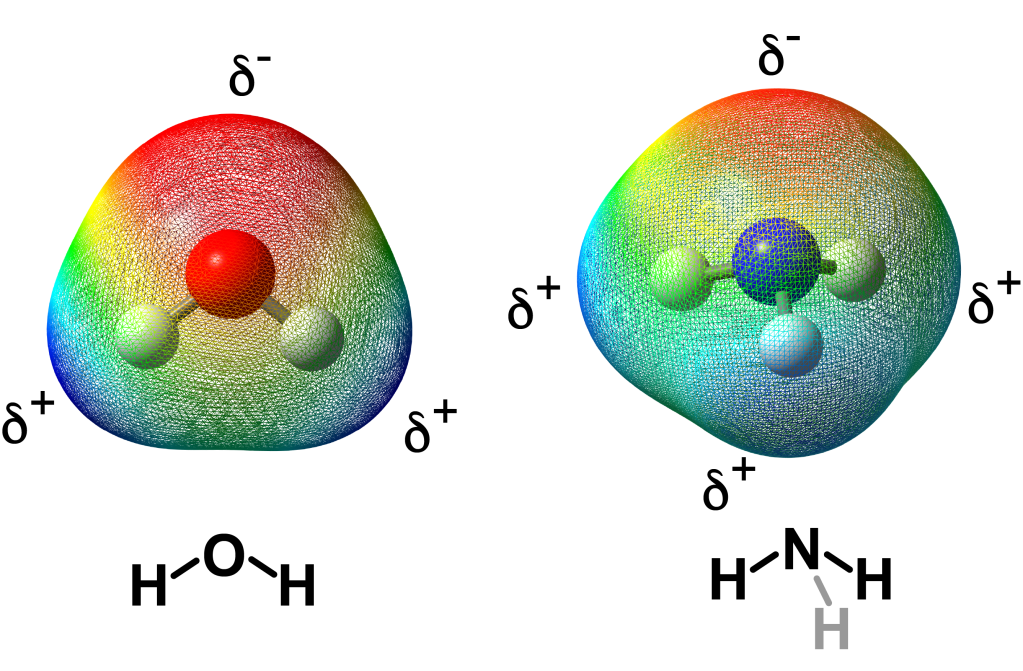
Computational model of electrostatic density maps of (left) water and (right) ammonia, in the gas phase.
The reaction of a metal hydride with water or ammonia releases hydrogen gas, termed hydrolysis. However, the reaction is extremely exothermic (releases energy, -277 kJ/mol). This release of energy, unless specifically controlled results the ignition of the released hydrogen, causing an explosion. Thus metal hydride must be strictly protected from contact with the atmosphere. A metal hydride hydrolysis chemical reaction is shown below.

However, chemical hydrides are different, as they contain both a hydride and a proton. Thus with the correct type of catalyst, these can be combined to release H2 and thus energy without excess energy. These chemical hydrides are much safer than metal hydrides.
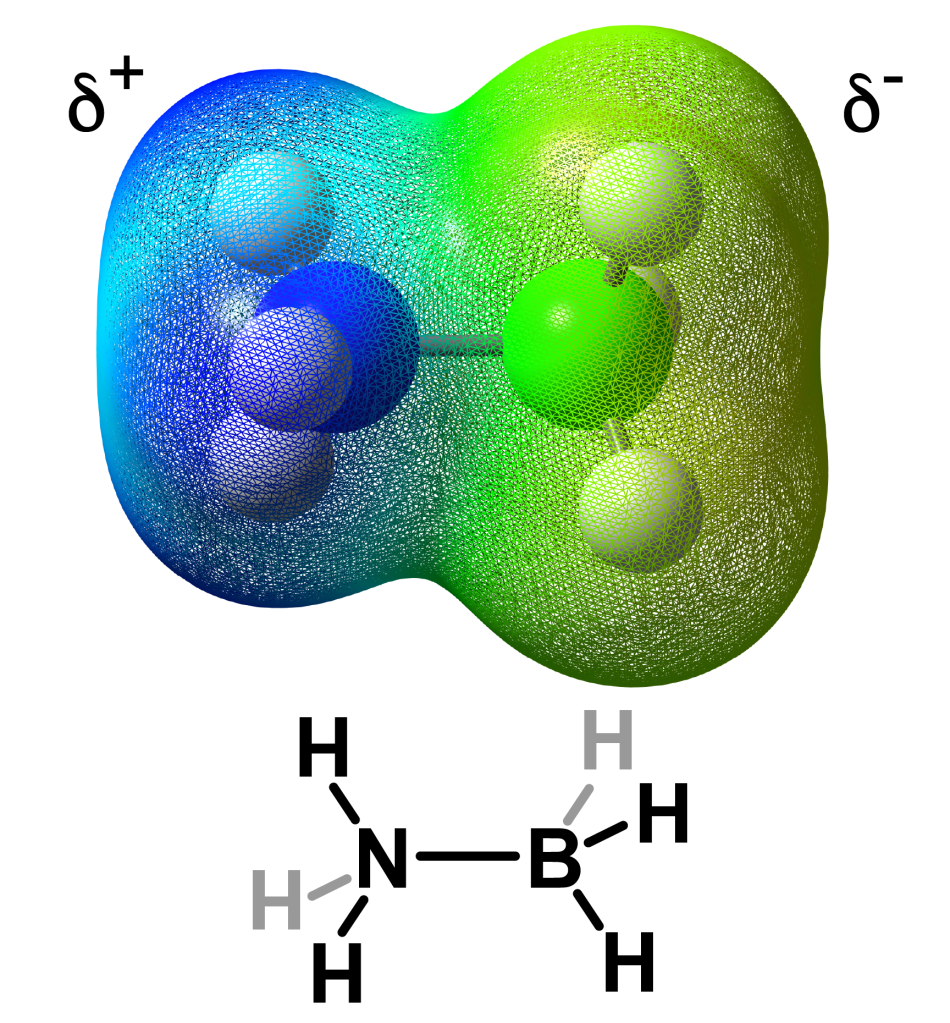
Computational model of electrostatic density maps of ammonia borane.
Ammonia borane is source of hydrogen in the RESR technology. Return to the ammonia borane page to read more.
